What is difference between anode and cathode. Link This is a common misconception.

How To Find Anode And Cathode In Diode Diode Black Rings Black Color
In a galvanic voltaic cell the anode is considered negative and the cathode is considered positive.
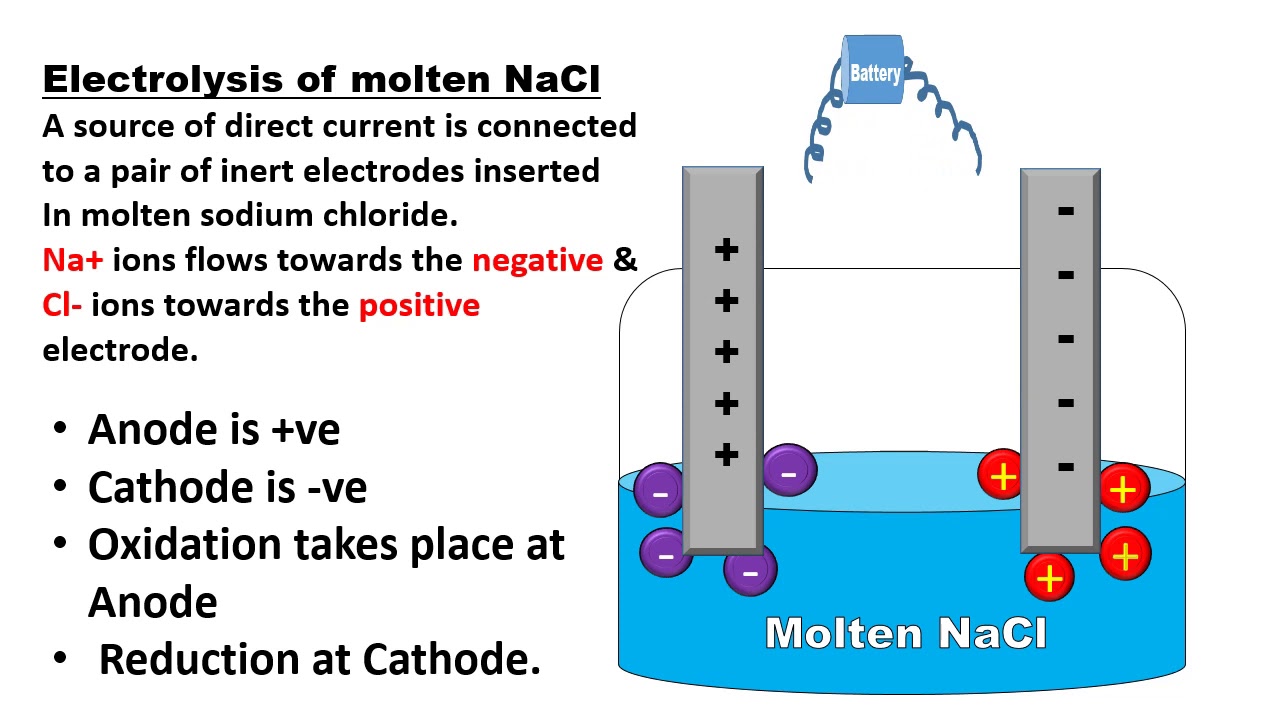
. However in an electrolytic cell the anode is taken to be positive whil. It is the positive electrode in an electrolytic cell while it is the negative electrode in a galvanic cell. The anode and cathode are defined by the flow of current.
This means the cathode is negatively charged the anode is positively charged. Connect one terminal of the plate to one positive terminal and the other one the negative terminal. The current on the anode is considered a positive current according to international convention.
For example in an electron tube electrons from the cathode travel across the tube toward the anode and in an electroplating cell negative ions are deposited at. They are so-called because the cations which are positively charged migrate to the negative cathode. A Cathode is a negative electrode whereas the anode is a positive electrode.
In an electrolytic cell the negative charge is on the cathode while the positive charge is on the anode. Advertisement In a galvanic cell electrons will move in to the anode. For goodness sakes man that answer is incomplete.
Is anode positive or negative in xray. Cathode and Anode Remember charge can flow either from positive to negative or from negative to positive. A terminal is not classified base on whether its positive or negative.
Here is where it gets interesting Electrolytic cell. Advertisement In a device which absorbs energy of charge such as recharging a battery the cathode is negative electrons flow out of the cathode and positive charge flows into it and in a device which provides energy such as battery in use the cathode is positive electrons flow into itRead More. Hence known as a cathode while the anions migrate to a positively charged anode and so-known as the anode.
What is anode in xray tube. In the general sense current refers to any movement of electrical charge. However in a galvanic cell even though electrons still move from the anode to the cathode it is defined as negative.
This seems reasonable as the anode is the source of electrons and cathode is where the electrons flow. In a galvanic voltaic cell the anode is considered negative and the cathode is considered positive. Its because the protons are attracted to the cathode so its mainly positive and therefore is positively charged.
Since electrons carry a negative charge then the anode is negatively charged. Is cathode positive or negative in galvanicRead More. It is a piece of metal shaped in the form of a bevelled disk with a diameter between 55 and 100 mm and.
It just happens that when a battery powers a circuit the cathode. The anode is usually considered to be negative. However in an electrolytic cell the anode is taken to be positive while the cathode is now negative.
So if electrons do the actual moving in a cell then current runs. Think of a large parallel capacitor. Is the anode positive or negative in a battery.
Answer 1 of 2. Why is an anode negative and a cathode positive. So for voltaic galvanic cell Anode is -ve while cathode is positive.
However in some experiments such as Gel Electrophoresis the anode is positive. Well if electrons are flowing from anode it has to be negative because the -ve charges will repel one another. The anode or anticathode is the component of the x-ray tube where x-rays are produced.
Anode the terminal or electrode from which electrons leave a system. However you should keep in mind the convention that current direction is according to where a positive charge would move not a negative charge. However in an electrolytic cell the anode is taken to be positive while the cathode is now negative.
In a battery or other source of direct current the anode is the negative terminal but in a passive load it is the positive terminal. This makes perfect sense according to my first reasoning. Which is the anode on an LED.
For example in an electron tube electrons from the cathode travel across the tube toward the anode and in an electroplating cell negative ions are deposited at the anode. In a battery or other source of direct current the anode is the negative terminal but in a passive load it is the positive terminal. For voltaic cell this is a spontaneous reaction process and you get a flow of electrons from anode to cathode.
This seems reasonable as the anode is the source of electrons and cathode is where the electrons flow. The capacitor plates would show a electrostatic charge. The electrode where oxidation occurs in an electrochemical cell.
The label anode or cathode is not defined with respect to the electrostatic sign as explained in the post by Maurice. Since an electrolytic cell requires energy to perpetuate the reaction we are pushing the electrons against their potential gradientIn an electrolytic cellelectrolytic cellAn electrolytic cell is an electrochemical cell that uses electrical energy to drive a non-spontaneous redox reaction. Similarly in diagrams comparing galvanic and electrolytic cells electrons move away from the anode to the cathode in the electrolytic cell making the anode positive.

Anodes And Cathodes Positive And Negative Bar Chart Mcat

Pin On Chem Redox Voltaic Galvanic Cell

Electrophoresis Chemistry Anode Cathode Diagram Chemistry Respiratory Therapy Study Materials

What Is Electrolytic Cell Electrochemistry Chemistry Basics Chemistry
0 Comments